Precision HealthCare Consultants (PHC) offers comprehensive clinical trial support services, leveraging advanced technology, data analytics, and specialized expertise to facilitate clinical trials both domestically and internationally. Their team comprises certified public health professionals and Certified Clinical Research Associates (CCRAs) with extensive experience in International Council for Harmonisation (ICH) guidelines, Standard Operating Procedures (SOPs), regulatory requirements, and Good Clinical Practices (GCPs).
I’m Vanessa Best, CEO of Precision Health Care Consultants. At Precision, we believe that everybody deserves equal access to healthcare, care without stigma or bias. We hold a vision to close the gap in health equity for underserved and marginalized populations worldwide. We provide comprehensive solutions across 11 countries, three continents, and 39 states. We’ve been instrumental in building capacity and infrastructure to combat diseases like HIV/AIDS and tuberculosis, making a global impact in clinical research monitoring, health science, policy development, and quality improvement. Our tangible difference lies in our expertise in insurance claims reviews, auditing, and independent dispute resolution. At Precision Health Care Consultants, we believe in the transformative power of healthcare to change lives. We’re here to partner with life sciences, pharmaceutical, and insurance companies to bridge the health equity gap and drive meaningful impact. Join us in our mission to protect and enhance the health and safety of humanity.
PHC’s team conducts on-site visits to clinical trial locations, performing activities such as:
Utilizing advanced technology, PHC performs remote monitoring to oversee clinical trial activities without the need for physical site visits. This approach allows for:
PHC conducts comprehensive regulatory reviews to ensure all trial activities comply with applicable laws, guidelines, and ethical standards. This includes:
PHC offers medical writing services to develop clear and accurate clinical trial documentation, including:
PHC manages the preparation and submission of required reports to regulatory authorities and stakeholders, ensuring:
PHC provides training programs for clinical trial staff, focusing on:
The Precision team assembles qualified personnel to efficiently meet donor-client satisfaction, provide unique assistance, clinical research monitoring, and administrative and compliance support services in accordance with USG and control labor laws.
Precision accurately monitors the quality of a portfolio of studies, we deploy the FDA-recommended Risk-Based Monitoring for clinical trials to identify and assess risks, and monitor and mitigate them. In addition, our team deploys a Process Tracing Performance-Based (PTPB) monitoring approach using step-by-step monitoring tools and indicators to classify issues with study procedures (e.g., IRB, protocol, consent, screening, enrolment, adverse events, etc.), determine follow-up actions, and ensure quality and overall ethical soundness of all monitored clinical studies.
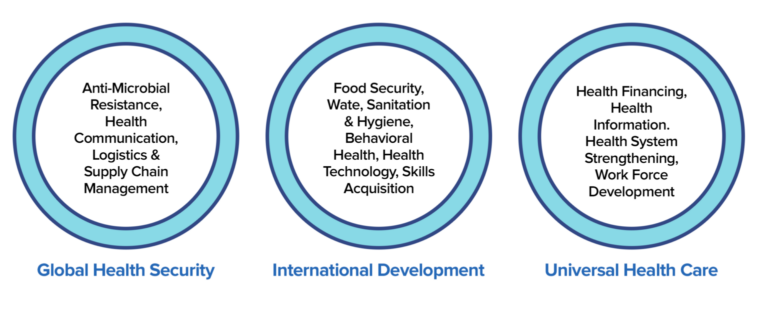
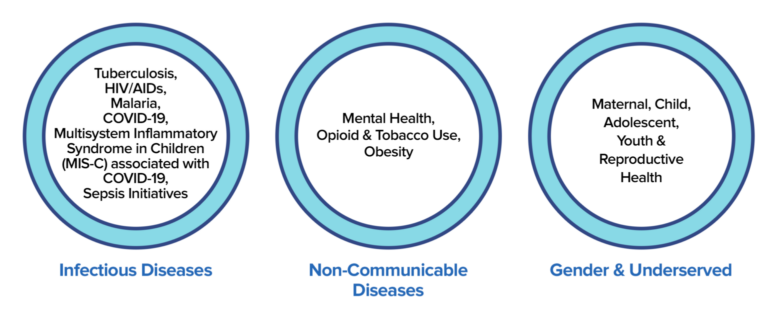
Broadly, we work in six areas: Infectious Diseases, Non-Communicable Diseases, Global Health Security, Gender& Underserved, International Development, and Universal Health Care.