Protocol/Clinical Trials Support
Get Expert Administrative Support Services for Your Protocol/Clinical Trials
Protocol/Clinical Trials Support
Our team's clinical trial experts use the latest technology, data, analytics, and expertise to create flexible, customized solutions to support your clinical trials here in the U.S. and internationally.
Precision provides a full range of protocol/clinical trial monitoring, visits, documentation, sound knowledge of regulatory standards, demonstrated experience in managing and monitoring all stages of clinical trials from protocol and associated document writing through enrollment, closeout, and generation of reports. Our experts are certified public health professionals, Certified Clinical Research Associates (CCRA), seasoned trainers with well-established local and regional experience in the International Council of Harmonization guidelines (ICH), SOPs, regulatory requirements, and Good Clinical Practices (GCPs).

Expertise Includes:
Research and Development
Clinical Research Design, Implementation & Monitoring
Technical Assistance & Training
Medical Writing, Coding, Review & Auditing
Private Sector Engagement
Monitoring, Evaluation, and Knowledge Management
Protocol monitoring services
Conducting on-site Visits
Conducting Remote monitoring Visits
Regulatory Review
Medical Writing
Report Submittal
Training
Core Areas and Tasks
Broadly, we work in six areas: Infectious Diseases, Non-Communicable Diseases, Global Health Security, Gender& Underserved, International Development, and Universal Health Care.
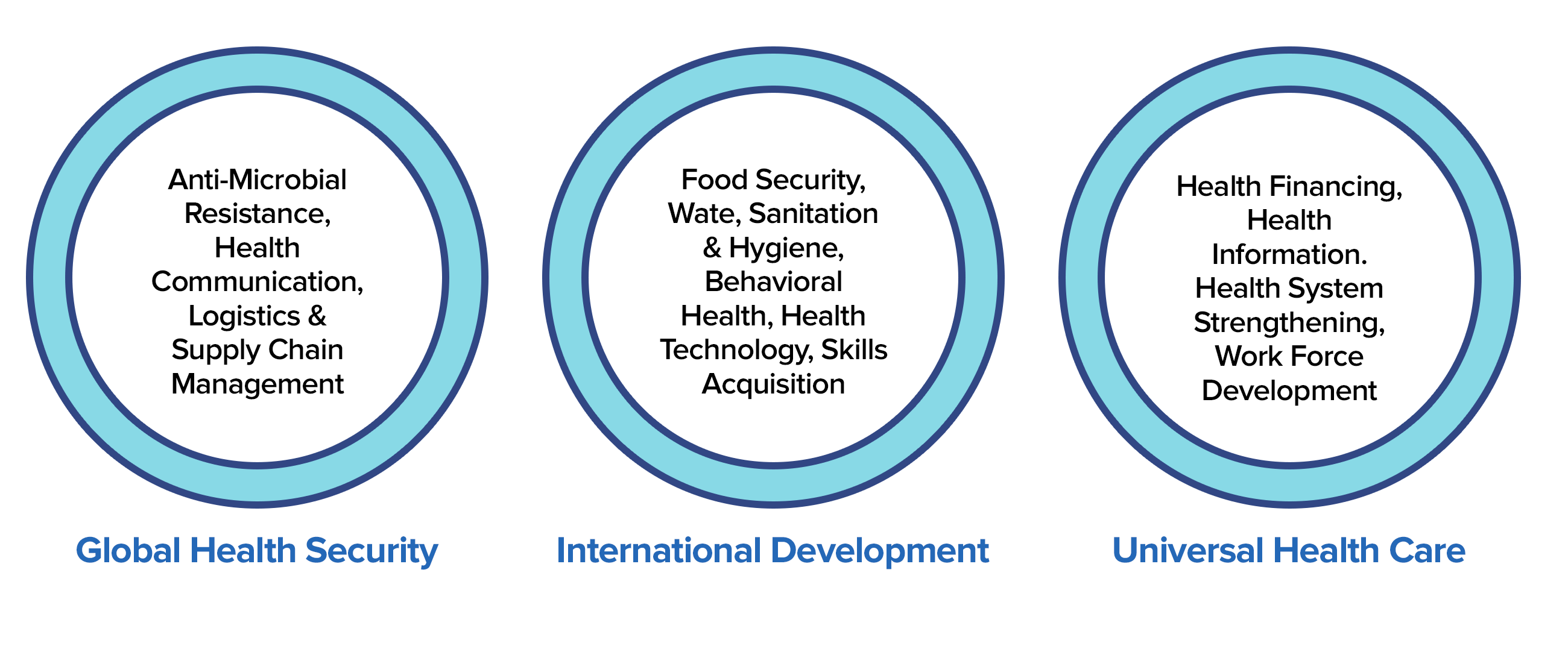
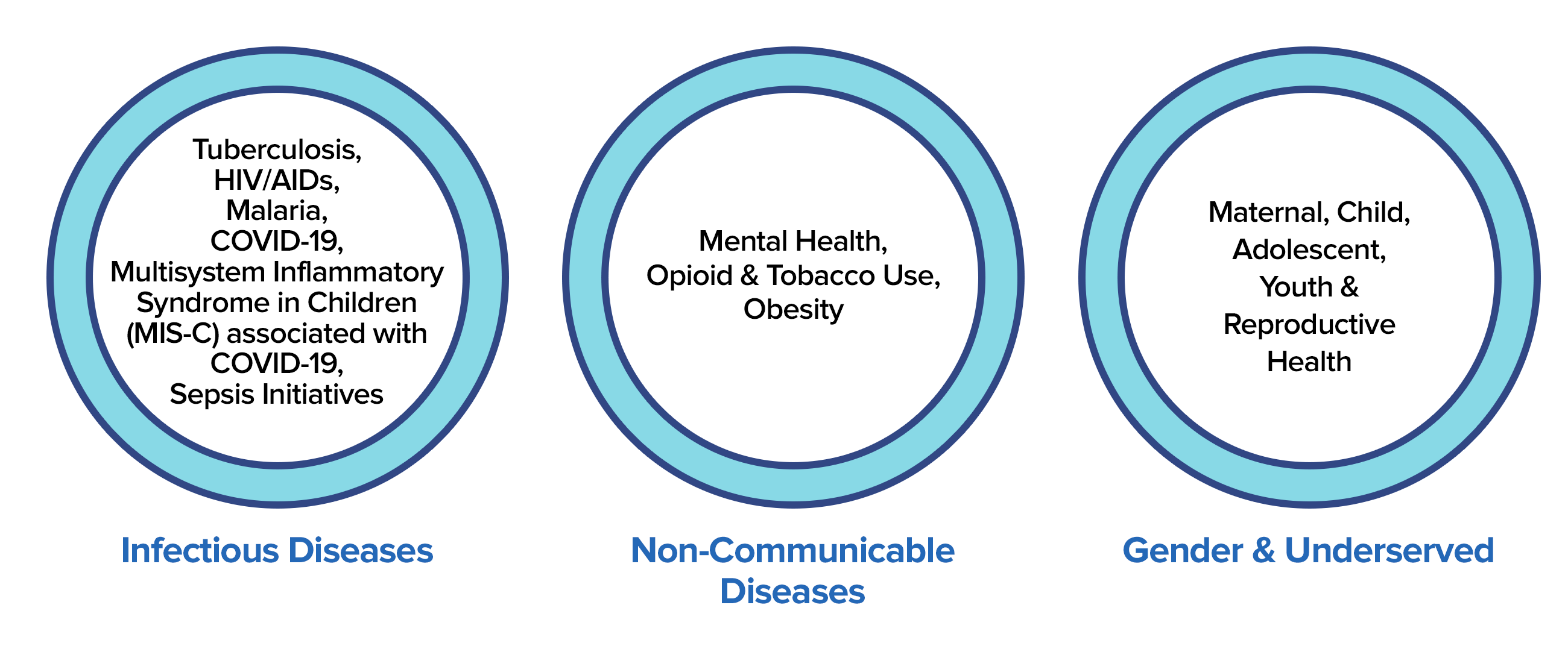
The Precision team assembles qualified personnel to efficiently meet donor-client satisfaction, provide unique assistance, clinical research monitoring, and administrative and compliance support services in accordance with USG and control labor laws.
Precision accurately monitors the quality of a portfolio of studies, we deploy the FDA-recommended Risk-Based Monitoring for clinical trials to identify and assess risks, and monitor and mitigate them. In addition, our team deploys a Process Tracing Performance-Based (PTPB) monitoring approach using step-by-step monitoring tools and indicators to classify issues with study procedures (e.g., IRB, protocol, consent, screening, enrolment, adverse events, etc.), determine follow-up actions, and ensure quality and overall ethical soundness of all monitored clinical studies.
Ready to get started?
Connect With Our Clinical Trial Support Team Today
